|
| Image credit: NASA |
The primary goal of the LH2 Era™ monthly webinar series is to provide accurate and actionable information to ensure robust, high performance, and operationally safe liquid hydrogen systems: https://sites.google.com/view/matthewemoran/training#h.cse56tn4ffmk
Meeting that goal requires assessing your current awareness about hydrogen systems and then leveling up by participating in the webinar. Take this poll to see where you are in an adapted version of the Gartner hype cycle: 1. Initial Exposure, 2. Inflated Expectations, 3. Disillusionment or Misinformation, 4. Pragmatic Productivity
Here's an interesting graphic of the hype cycle: https://en.wikipedia.org/wiki/Gartner_hype_cycle#/media/File:Hype-Cycle-General.png
Not necessarily followed by all technologies (or even most), and not generally applicable to hydrogen since it's been around a very long time. Interesting to compare though...
Question 2:
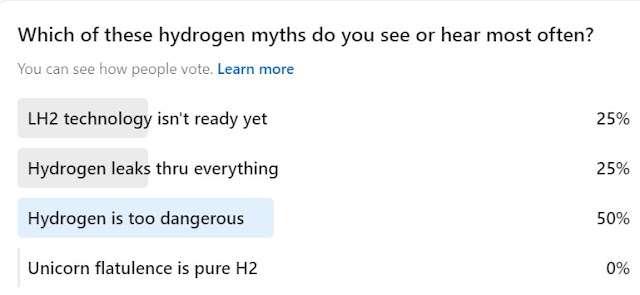
The first three myths are addressed here: https://blog.matthewemoran.com/2022/04/hydrogen-myth-busting-episode-1.html
I can neither confirm nor deny the unicorn answer, but there seems to be a consensus on glitter and rainbows on the internet. And that's how the first three myths probably got started too...
Question 3:
300 series stainless steel is the most common structural material used for all types of hydrogen service. However, note that 400 series stainless steel has a ductile to brittle transition temperature (DBTT) above liquid hydrogen temperature so is generally not suitable for cryogenic service in this range.
Aluminum alloys are historically the second most common structural material for hydrogen service. Some very recent research papers are suggesting there may be some hydrogen embrittlement possible at very high pressures. I've never witnessed nor heard of this issue in any operational system, but something to stay up to date on as more data becomes available.
Teflon can be used as a seal material or gasket for liquid hydrogen service since it still cold flows at these temperatures. Other seal material options are also appropriate depending on the application.
Cotton canvas infused with highly flammable chemicals was used for hydrogen containment in an infamous dirigible more than 85 years ago. This is an inappropriate material choice for any lifting gas (hydrogen, helium, hot air, etc.) since an electric discharge can set the entire structure aflame: https://blog.matthewemoran.com/2022/04/hydrogen-myth-busting-episode-1.html
Question 4:
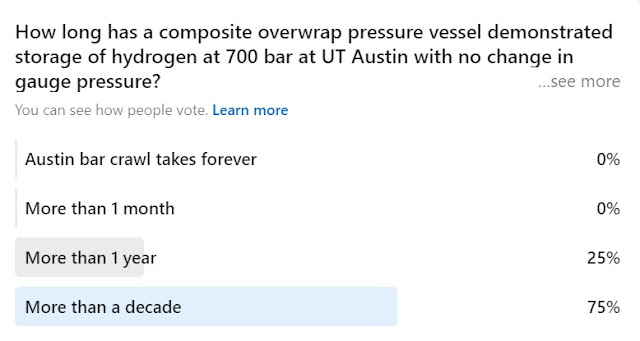
The answer to this one is over a decade as of the time I visited UT Austin several years ago. This of course speaks to the myth about hydrogen leaking though everything. Methods and materials to mitigate hydrogen leaks are very well established and have been operationally validated with liquid hydrogen systems in continuous use since the 1960s.
The first answer is not relevant but nonetheless also true. I did a bar (pub) crawl with my oldest son years ago when he lived there during the city's annual SXSW festival. We gave up before reaching the last stop but had a great time :)
Question 5:
I've been driving my battery electric vehicle (BEV) for 18 months sitting on top of a lithium-ion battery pack with this quantity of energy storage. But if live rounds were being fired at it (or the battery compartment was damaged in a collision) I'd abandon it and get far away as quickly as possible. Li-ion batteries are subject to thermal runaway if any of the cells are crushed or penetrated, resulting in that energy being rapidly released and leaving behind a smoldering melted mass.
For the fossil fuel options, there are at least three serious hazards in this scenario: 1. ignition (conflagration or detonation); 2. inhalation of toxic fumes or smoke; 3. asphyxiation. The liquid fuels also spread quickly along the ground if the containment is breached, significantly increasing the physical area of risk exposure. Propane is heavier than air so it increases the physical volume of risk exposure. Natural gas is lighter than air so will eventually dissipate unless confined. Both gases pose a significant detonation hazard in this scenario.
In the case of compressed gaseous hydrogen, this scenario has already been tested on composite overwrap (COPV) pressure vessels (without the bystander). From the conclusion section of the test report: "When penetrated by AP [armor piercing] rounds, the cylinder will vent quickly without producing a flame. The cylinder will not catastrophically fail when pierced by a round, retaining its structural integrity and venting its contents safely."
The full report can be accessed here: http://www.powersourcesconference.com/Power%20Sources%202018%20Digest/docs/P10-6.pdf
Also note that hydrogen is not toxic, and is actually used as a breathing gas with oxygen (hydrox) for very deep diving. At ambient temperature in air, it rises at a rate of 20 m/s. And it produces no smoke nor soot when it burns.
Liquid hydrogen (LH2) was not tested in the cited report. LH2 in this scenario would vaporize very quickly if the containment vessel is breached and begin rising as it quickly warms up. Liquefied natural gas (LNG) would behave similarly, but would vaporize and rise much more slowly than LH2.
Question 6:
Section 3.3 of the passive Cryogenic Fluid Management report provides a detailed comparison of these options. See: https://sites.google.com/view/matthewemoran/training#h.p_0kmGtNXJhZlO
The most common insulation historically for vacuum-jacketed dewar tanks is perlite powder. It has been used in many over-the-road truck trailer vessels and stationary vessels including the large LH2 spherical dewar tank built in the 1960s that supported the Apollo and space shuttle programs. It is comparatively inexpensive and has moderate thermal performance in a hard vacuum in comparison to the other options. However, perlite has a tendency to settle over time and uncover the upper portions of the vacuum space thereby increasing the heat load.
Glass microspheres have slightly better thermal performance than perlite and are not as prone to settling. They are also relatively inexpensive and were used on the new spherical LH2 dewar tank that is 50% larger than the previous tank used for space shuttle launches.
Aerogel beads or blankets do not perform as well as perlite or glass microspheres in a hard vacuum jacket, but are better performing than those options at soft vacuum or atmospheric pressure. Aerogel insulation is more costly but may be a suitable choice for situations where thermal performance is needed in varying atmospheric and vacuum environments (e.g., a tank that is launched in normal atmosphere and then is in the vacuum of space for some period of time).
Multilayer insulation (MLI) is the best performing option in a hard vacuum jacket. It is comprised of reflective thermal shields separated by low conductivity high porosity spacer materials (e.g., double aluminized mylar with dacron net spacers). The thermal performance of MLI is affected by a number of parameters including appropriate installation and close-outs. It is the most expensive of the options shown, and is used in high performance vacuum-jacketed stationary storage tanks and various types of tanks in spacecraft applications.
Spray on foam (SOFI) provides moderate thermal performance in a normal atmosphere but has very poor performance in vacuum conditions. It is among the most inexpensive options and has been used on launch vehicle tanks and the space shuttle external tank. SOFI is not generally appropriate for stationary LH2 tanks but can be a suitable option for higher temperature cryogens depending on the use case (e.g., methane, oxygen, nitrogen, etc.).